Chlorine And Bromine Bond Type . for brcl3, a single shared covalent bond for each is formed between the bromine atom and the 3 chlorine atoms. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. bromination vs chlorination: let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs. Therefore each na becomes a na + cation. draw the lewis structure that represents the compound that is formed when carbon and chlorine bond with one another. for example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. Lastly, we will determine the.
from solvedlib.com
Therefore each na becomes a na + cation. for brcl3, a single shared covalent bond for each is formed between the bromine atom and the 3 chlorine atoms. Lastly, we will determine the. for example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. draw the lewis structure that represents the compound that is formed when carbon and chlorine bond with one another. bromination vs chlorination: let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine.
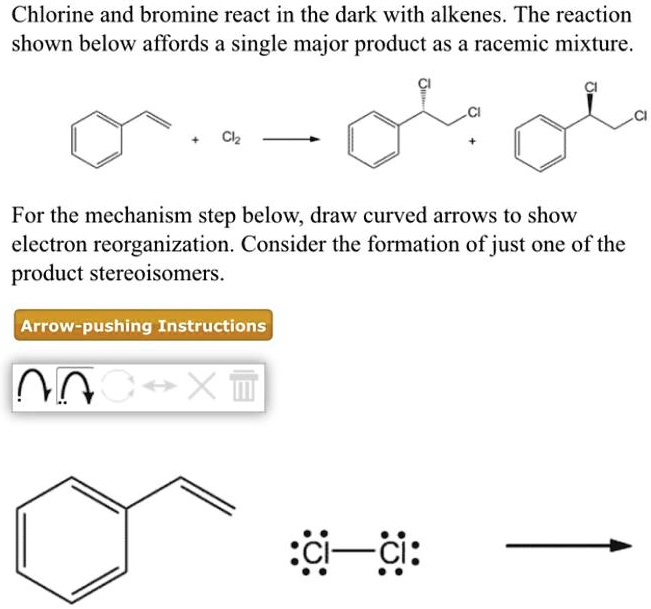
Chlorine and bromine react in the dark with alkenes. … SolvedLib
Chlorine And Bromine Bond Type Therefore each na becomes a na + cation. for brcl3, a single shared covalent bond for each is formed between the bromine atom and the 3 chlorine atoms. let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs. bromination vs chlorination: for example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. Lastly, we will determine the. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. draw the lewis structure that represents the compound that is formed when carbon and chlorine bond with one another. Therefore each na becomes a na + cation.
From www.differencebetween.com
Difference Between Bromine and Chlorine Compare the Difference Chlorine And Bromine Bond Type when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. draw the lewis structure that represents the compound that is formed when carbon and chlorine bond with one another. let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that. Chlorine And Bromine Bond Type.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Chlorine And Bromine Bond Type bromination vs chlorination: for brcl3, a single shared covalent bond for each is formed between the bromine atom and the 3 chlorine atoms. draw the lewis structure that represents the compound that is formed when carbon and chlorine bond with one another. for example, in the reaction of na (sodium) and cl (chlorine), each cl atom. Chlorine And Bromine Bond Type.
From www.thoughtco.com
Examples of Ionic Bonds and Ionic Compounds Chlorine And Bromine Bond Type Lastly, we will determine the. draw the lewis structure that represents the compound that is formed when carbon and chlorine bond with one another. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. let's illustrate how a covalent bond forms between iodine and. Chlorine And Bromine Bond Type.
From blog.chloramineconsulting.com
Chlorine vs. Bromine in Indoor Pools Chlorine And Bromine Bond Type draw the lewis structure that represents the compound that is formed when carbon and chlorine bond with one another. for brcl3, a single shared covalent bond for each is formed between the bromine atom and the 3 chlorine atoms. bromination vs chlorination: for example, in the reaction of na (sodium) and cl (chlorine), each cl atom. Chlorine And Bromine Bond Type.
From www.buzzle.com
Bromine Vs. Chlorine Chlorine And Bromine Bond Type for brcl3, a single shared covalent bond for each is formed between the bromine atom and the 3 chlorine atoms. Therefore each na becomes a na + cation. let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs. for example, in the reaction of na (sodium) and cl. Chlorine And Bromine Bond Type.
From wisc.pb.unizin.org
Resonance Structures and Formal Charge (M8Q3) UWMadison Chemistry Chlorine And Bromine Bond Type Therefore each na becomes a na + cation. let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. bromination vs chlorination: draw the lewis. Chlorine And Bromine Bond Type.
From slideplayer.com
Chemical Bonds. ppt download Chlorine And Bromine Bond Type bromination vs chlorination: draw the lewis structure that represents the compound that is formed when carbon and chlorine bond with one another. for example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. let's illustrate how a covalent bond forms between iodine and bromine, with the. Chlorine And Bromine Bond Type.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison Chlorine And Bromine Bond Type for example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. for brcl3, a single shared covalent bond for each is formed between the bromine atom and the 3 chlorine atoms. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the. Chlorine And Bromine Bond Type.
From slideplayer.com
Chapter 6 Chemical Bonding. ppt download Chlorine And Bromine Bond Type Therefore each na becomes a na + cation. draw the lewis structure that represents the compound that is formed when carbon and chlorine bond with one another. let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs. for example, in the reaction of na (sodium) and cl (chlorine),. Chlorine And Bromine Bond Type.
From www.researchgate.net
Oxidative addition of chlorine and bromine to complex 3 (above) and Chlorine And Bromine Bond Type Therefore each na becomes a na + cation. let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs. draw the lewis structure that represents the compound that is formed when carbon and chlorine bond with one another. for example, in the reaction of na (sodium) and cl (chlorine),. Chlorine And Bromine Bond Type.
From slideplayer.com
Chapter 5 Alkene Addition Reactions ppt download Chlorine And Bromine Bond Type Therefore each na becomes a na + cation. let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs. bromination vs chlorination: draw the lewis structure that represents the compound that is formed when carbon and chlorine bond with one another. Lastly, we will determine the. for brcl3,. Chlorine And Bromine Bond Type.
From www.youtube.com
How To Easily Draw The Mass Spectra Of Chlorine And Bromine Edexcel Chlorine And Bromine Bond Type draw the lewis structure that represents the compound that is formed when carbon and chlorine bond with one another. let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs. Therefore each na becomes a na + cation. Lastly, we will determine the. when chlorine (as a gas or. Chlorine And Bromine Bond Type.
From kpu.pressbooks.pub
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Chlorine And Bromine Bond Type Therefore each na becomes a na + cation. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Lastly, we will determine the. for brcl3, a single shared covalent bond for each is formed between the bromine atom and the 3 chlorine atoms. let's. Chlorine And Bromine Bond Type.
From www.slideserve.com
PPT Types of Bonding and Lewis Structures PowerPoint Presentation Chlorine And Bromine Bond Type for brcl3, a single shared covalent bond for each is formed between the bromine atom and the 3 chlorine atoms. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Lastly, we will determine the. let's illustrate how a covalent bond forms between iodine. Chlorine And Bromine Bond Type.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine And Bromine Bond Type when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. bromination vs chlorination: Lastly, we will determine the. for example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. draw the lewis structure. Chlorine And Bromine Bond Type.
From slideplayer.com
Determine the Bond Type (Electronegativities) ppt download Chlorine And Bromine Bond Type draw the lewis structure that represents the compound that is formed when carbon and chlorine bond with one another. bromination vs chlorination: when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Lastly, we will determine the. for example, in the reaction of. Chlorine And Bromine Bond Type.
From www.youtube.com
Difference Between Bromine and Chlorine YouTube Chlorine And Bromine Bond Type let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs. for brcl3, a single shared covalent bond for each is formed between the bromine atom and the 3 chlorine atoms. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes. Chlorine And Bromine Bond Type.
From www.alamy.com
Fluorine, Bromine and Chlorine Molecular Model of Atom. Vector Chlorine And Bromine Bond Type Therefore each na becomes a na + cation. let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs. for example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. when chlorine (as a gas or dissolved in water). Chlorine And Bromine Bond Type.